Cancer cachexia drug, TCMCB07, has started an innovative Phase 2
trial in 2025
Cachexia is a metabolic syndrome characterized by an uncontrollable loss in body mass. It is a common comorbidity for patients with cancer and other chronic conditions.
Cachexia decreases patient quality of life by causing debilitating fatigue, weakness, and reduced mobility.
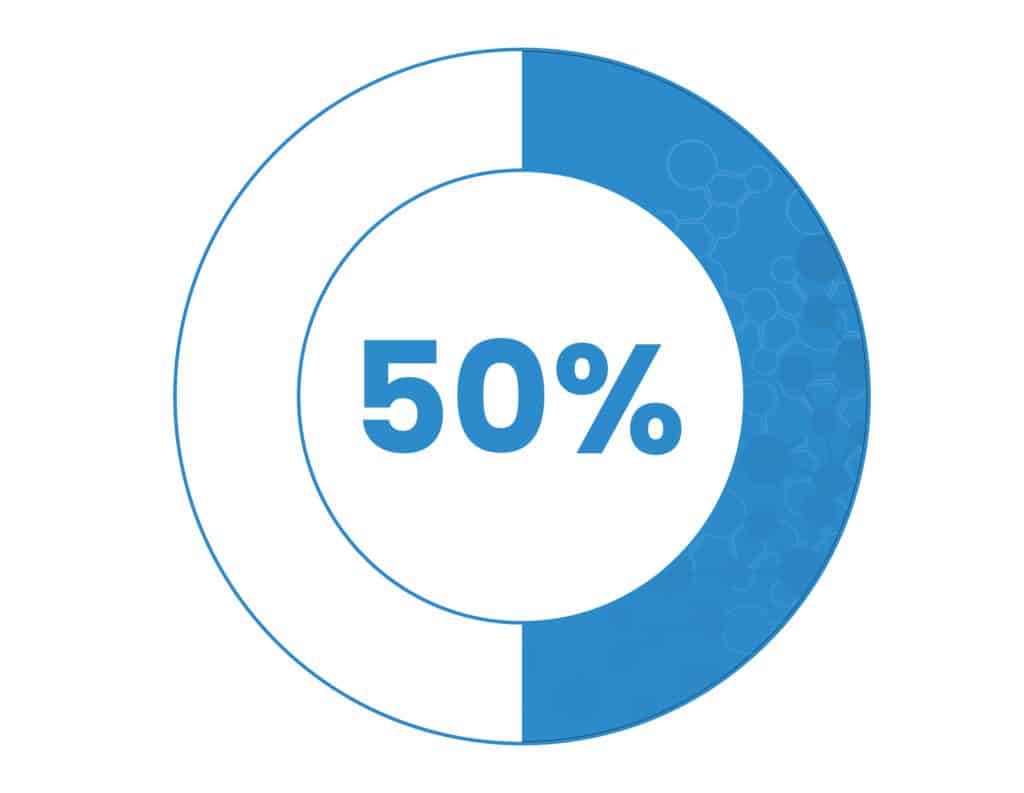
50% of diagnosed cancer patients are affected by cachexia each year.1
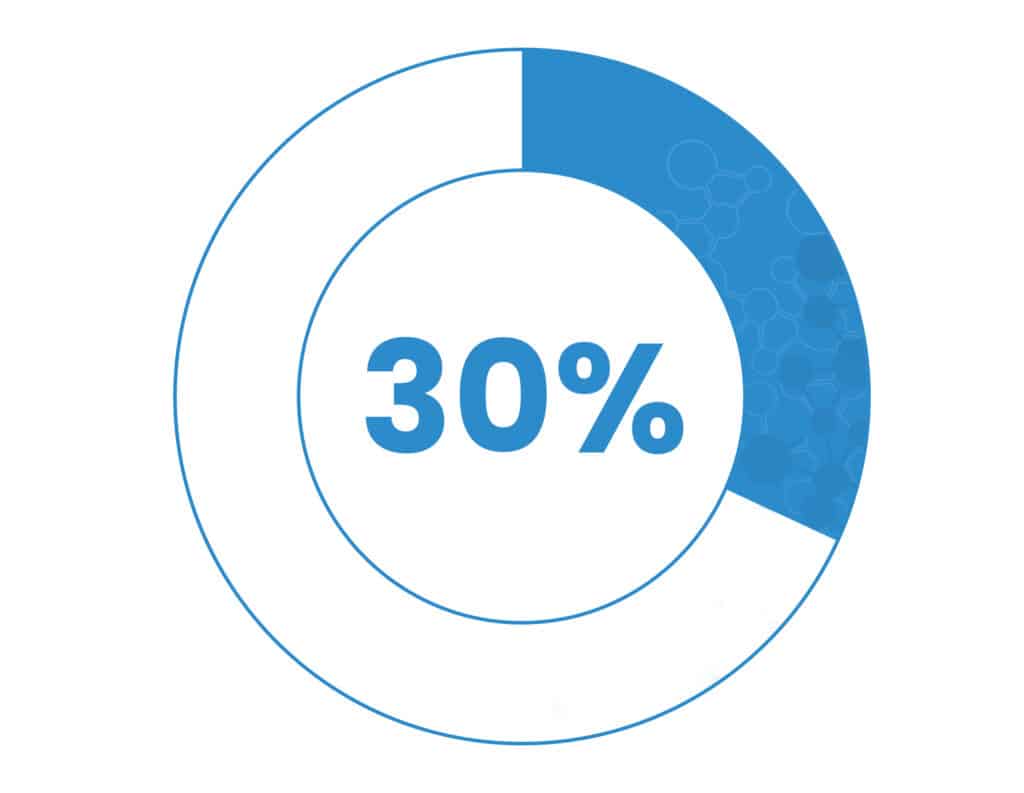
Up to 30% of deaths in late-stage cancer patients are caused by cachexia.2
There are currently no clinically-approved, efficacious
drugs treating cachexia in the United States.2 Endevica is changing that.
Treatment that’s proactive, not reactive3
This is an innovative, prophylactic treatment paradigm aimed at improving health outcomes and obviating the traditional approach being used by competitors.
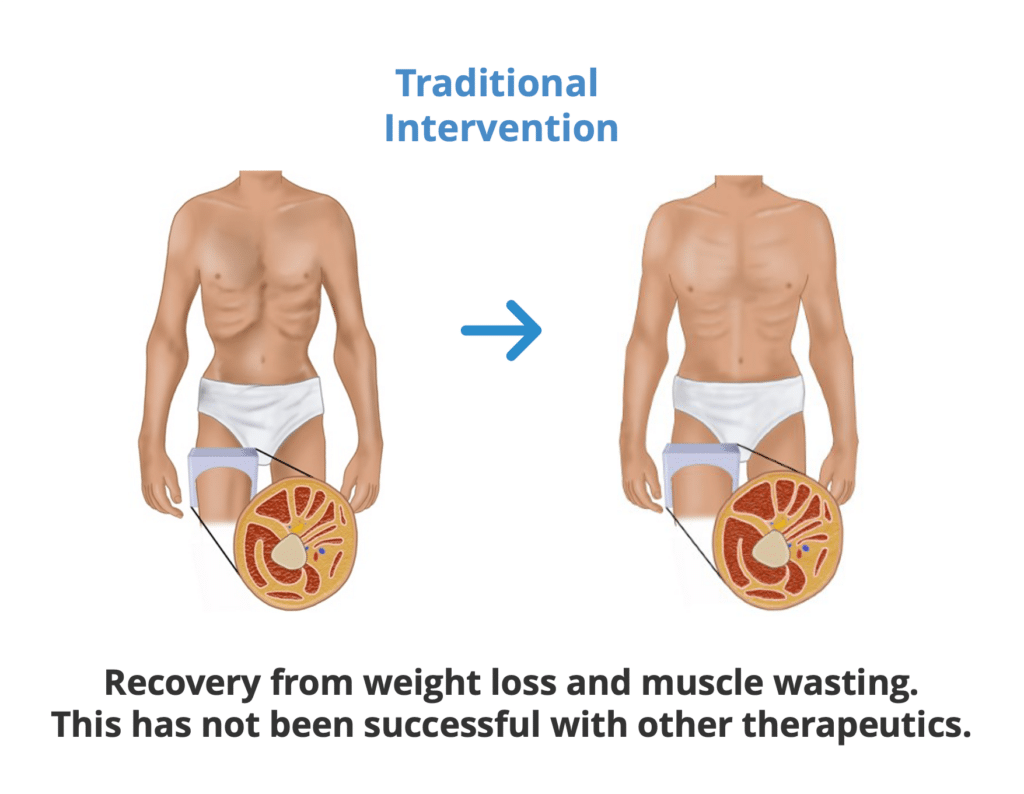
Traditional Treatment Approach
- Cachexia is treated upon diagnosis in a reactive manner. After patients regain weight, they are taken off treatment and lose weight again.
- Patient suffers clinical harm from suboptimal approach.
Prophylactic Treatment Approach
- Cachexia is prevented with adjuvant treatment in newly-diagnosed cancer patients, maintaining a healthy body weight before extensive mass loss has occurred.4
- By treating newly-diagnosed cancer patients, the weight gain caused by TCMCB07 could improve patients’ outcomes.5

Advantages of TCMCB07
Poised for Growth, Clear Milestones Ahead
Phase 2 innovative trial begins
Phase 2 top-line readouts expected
Phase 3 pivotal trial begins
Expanded Access Policy
Expanded Access, also sometimes called Compassionate Use, refers to the FDA’s pathway in which patients with serious or life-threatening diseases or conditions may gain access to an investigational therapy outside of a clinical trial evaluating safety or efficacy.
At this time, Endevica Bio does not make its investigational drugs available prior to regulatory approval.
We are now enrolling patients and conducting clinical trials to gain regulatory approvals for TCMCB07. We believe this approach will allow us to bring our therapies to as many patients in need as possible. Information on all ongoing investigational trials sponsored by Endevica Bio can be found at clinicaltrials.gov.
As authorized by the 21st Century Cures Act, Endevica Bio may revise this expanded access policy at any time. Additionally, the posting of this policy by Endevica Bio shall not serve as a guarantee of access to any specific investigational drug by any individual patient.
1. Mariean et al. Cancers. 2023.
2. Cleveland Clinic. (n.d.). Cancer cachexia. https://my.clevelandclinic.org/health/diseases/cancer-cachexia
3. https://www.biospace.com/press-releases/endevica-bio-to-start-phase-2-clinical-trial-of-drug-to-prevent-weight-loss-in-cancer-patients
4. https://ascopubs.org/doi/10.1200/JCO.2023.41.16_suppl.e15195
5. Lennon et al. Curr Oncol Rep. 2016. and Zadeh et al. JCSM. 2013.